Limbal Stem Cells Deficiency, Etiologies, Clinical Manifestations and Management
Ophthalmologist, Cornea Specialist
Pacific Eye Institute, California
INTRODUCTION
Ocular surface cells do not live forever, they shed off, new cells will replace old, damaged or dead cells. Limbal epithelial stem cells are the ultimate source of corneal epithelial cell regeneration. Studies show that corneal stem cells reside in the limbus. “Palisades of Vogts” that are equivalent to the rete ridge in skin, are known as the primary location of the limbal stem cells in the human eye. Limbal stem cells, like many other stem cells, have a long-life span, slowly proliferate and differentiate to the corneal epithelial cells. Thoft and Friend first introduced the XYZ model of corneal epithelial maintenance (Figure 1). In this model, after shedding the old cells (Z), new cells move and differentiate from limbus to the center of the cornea (Y) and from deep layer of epithelial cells to the superficial layers (X). (1,2)
Limbal stem cells are critical to renew ocular surface and maintain corneal clarity. They keep the normal cornea clear and avascular, through a phenomenon which is known as limbal barrier. When function of theses stem cells is compromised, the cornea usually gets vascularized and opacified, that based on the extent and severity, can cause profound visual impairment.
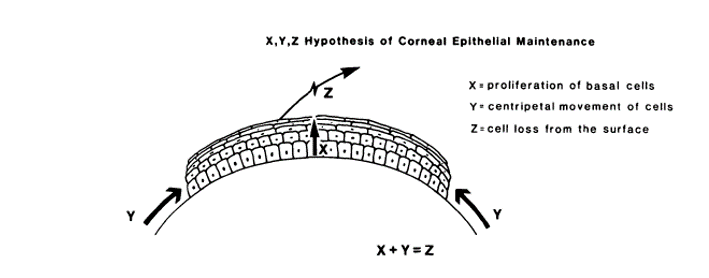
Figure 1, The X, Y, Z hypothesis of corneal epithelial maintenance. (From Invest Ophthalmol Vis Sci. 1983 Oct;24(10):1442-3.Thoft RA, Friend J.)
CAUSES OF LIMBAL STEM CELL DEFICIENCY
Limbal stem cell deficiency (LSCD) can develop in different settings, such as traumatic, immunologic, or genetic diseases that affect the ocular surface. Chemical injuries, including acid or base burns, can cause LSCD. Immunologic diseases such as Ocular Cicatricial Pemphigoid and Stevens-Johnson syndrome may lead to LSCD. Some genetic conditions like congenital aniridia can cause LSCD. List of most important causes of LSCD are summarized in Table 1. Even long-term contact lens wear may lead to LSCD. (3-5)
Table 1, Causes of limbal stem cell deficiency
Hereditary
- Anirida
- Keratitis associated with multiple endocrine deficiency
- Epidermal dysplasia (ectrodactyly-ectodermal dysplasia-clefting syndrome, KID syndrome)
Acquired
- Chemical or thermal burns
- Stevens-Johnson syndrome, toxic epidermal necrolysis
- Multiple surgeries or cryotherapies to limbus
- Contact lens-induced keratopathy
- Severe microbial infection extending to limbus
- Antimetabolite uses (5-FU, mitomycin C)
- Radiation
- Chronic limbitis (vernal, atopy, phlyctenular)
- Mucous membrane pemphigoid
CLINICAL MANIFESTATION OF LIMBAL STEM CELL DEFICIENCY
Early stages of LSCD can be present with unstable tear film, along with signs and symptoms of dry eye that are hard to control. Haze and irregular epithelium are frequently seen in LSCD. A non-healing corneal epithelial defect might be due to LSCD. Ultimately, corneal vascularization and opacification can happen in severe cases of LSCD. (3-5)
Here is the summarized list of the clinical signs of LSCD:
- Loss of limbal anatomy
- Irregular, thin epithelium
- Stippled fluorescein staining of the area covered by abnormal epithelium
- Filaments and erosions
- Superficial and deep corneal vascularization
- Persistent epithelial defects leading to ulceration, melting, and perforation
- Fibrovascular pannus on cornea
- Ocular surface scarring, keratinization, and calcification
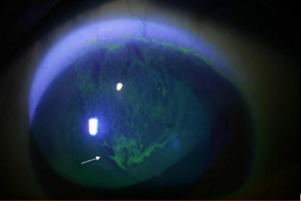
Below, Figure 1 shows a sever and total LSCD, that caused totally vascularized and opacified cornea. Figure 2 shows a milder case of LSCD, partial deficiency after long-term contact lens wear.
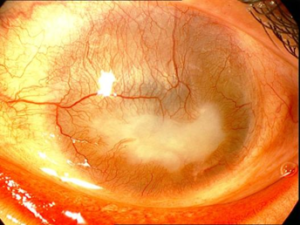
Figure 2. Total LSCD after alkaline burn, showing corneal vascularization and opacification.
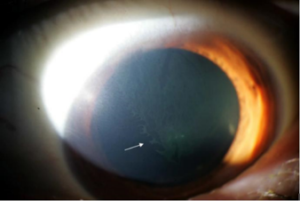
Figure 3. Partial LSCD after chronic and long-term soft contact lens wear. Pictures show irregular and hazed epithelium (upper) and late fluorescein staining of the irregular epithelium (lower).
MEDICAL MANAGEMENT OF LIMBAL STEM CELL DEFICIENCY
Optimization of the ocular surface health is usually the first step in the treatment of LSCD. That includes treatment of dry eye, through preservative-free artificial tears, punctal plugs and topical cyclosporine. Ocular surface inflammation mostly accompanies LSCD, especially in the acute and sub-acute stages, and in cases of Stevens-Johnson’s syndrome and chemical burn. Topical corticosteroids are usually necessary to control the inflammation. Intraocular pressure should be monitored, since glaucoma is very common in the setting of LSCD, especially after chemical injuries. Glaucoma management sometimes needs to be addressed surgically. With any epithelial defect, in addition to appropriate preventive antibiotic coverage, a bandage contact lens is helpful. (4-7)
SURGICAL MANAGEMENT OF LIMBAL STEM CELL DEFICIENCY
LSCD based on the extent of involvement is categorized to total, when the entire limbus is severely damaged, and sub-total. The course is usually categorized as acute, sub-acute and chronic. In severe cases, many patients would need surgical intervention in the acute, sub-acute and chronic phase. Target in acute and sub-acute stage of LSCD is usually to help a persistent epithelial defect and preventing symblepharon formation by amniotic membrane graft, superficial keratectomy, and sometimes tarsorrhaphy. In the chronic phase, mostly surgical procedure targets ocular surface reconstruction, including fornices reconstruction, limbal stem cell transplantation, and eyelid surgery in cases of chemical injuries and cicatricial diseases. In some diseases like aniridia, the course is chronic and insidious. (7-10)
In the acute phase of the LSCD, mostly in chemical injuries and Stevens-Johnson syndrome, a surgical approach is used to address a persistent corneal epithelial defect or to cover the injured ocular surface to prevent symblepharon. For non-healing corneal epithelial defect, a bandage contact lens amniotic membrane disc is usually the first step. Nonresponding cases of persistent corneal epithelial defects in the setting of LSCD, usually need Amniotic Membrane Transplantation and tarsorrhaphy. Long standing corneal epithelial defects may lead to corneal melt and perforation, that might need tectonic of patch corneal transplant.
Based on the extent of involvement, some cases of total or sub-total LSCD ultimately would need replacing the limbal stem cells by transplantation. Limbal stem cell transplantation could be autograft, from the fellow eye, or allograft, from donor tissue. Allograft transplantation, like keratolimbal allograft (KLAL) requires systemic immunosuppression after surgery. After replacing limbal stem cells, sometimes corneal transplantation is required due to severe corneal scaring and vascularization. (8-14). Cultivated stem cell transplantation has been tried successfully in some cases of LSCD. In this procedure ex vivo expansion of stem cells on a fibrin or amniotic membrane as the carrier of cells is used and the source of the cells are the fellow eye in unilateral cases. The main advantage of this method is providing an autologous source of limbal stem cells, to avoid an allograft that would require immunosuppression. Artificial cornea or keratoprosthesis is another option that can open a clear window in such vascularized and opacified cornea. There are several types of keratoprosthesis available that have been used with variable success. Boston Keratoprosthesis (MEEI, Boston) is the one that mostly is used in the United States. (3-5,15).
REFERENCES
- Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442–3.
- Kinoshita S, Kiorpes TC, Friend J, Thft RA. Limbal epithelium in ocular surface wound healing. Invest Ophthalmol Vis Sci. 1982;23:73–80.
- Presentation, diagnosis and management of limbal stem cell deficiency.Sejpal K, Bakhtiari P, Deng SX.Middle East Afr J Ophthalmol. 2013 Jan-Mar;20(1):5-10.
- Update on limbal stem cell transplantation. Bakhtiari P, Djalilian A.Middle East Afr J Ophthalmol. 2010 Jan;17(1):9-14.
- Medically reversible limbal stem cell disease: clinical features and management strategies. Kim BY, Riaz KM, Bakhtiari P, Chan CC, Welder JD, Holland EJ, Basti S, Djalilian AR.Ophthalmology. 2014 Oct;121(10):2053-8.
- Santos MS, Gomes JA, Hofling-Lima AL, Rizzo LV, Romano AC, Belfort R., Jr Survival analysis of conjunctival limbal grafts and amniotic membrane transplantation in eyes with total limbal stem cell deficiency. Am J Ophthalmol. 2005;140:223–30.
- Holland EJ, Schwartz GS. Changing concepts in the management of severe ocular surface disease over twenty-five years. Cornea. 2000;19:688–98.
8.. Dogru M, Tsubota K. Current concepts in ocular surface reconstruction. Semin Ophthalmol. 2005;20:75–93.
9.. Holland EJ, Schwartz CS. The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea. 1996;15:549–56.
- Shimazaki J, Aiba M, Goto E, Kato N, Shimmura S, Tsubota K. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109:1285–90.
- Tsai RJ, Tseng SC. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994;3:389–400.
- Tsubota K, Toda I, Saito H, Shinozaki N, Shimazaki J. Reconstruction of the corneal epithelium by limbal allograft transplantation for severe ocular surface disorders. Ophthalmology. 1995;102:1486–95.
13.. Espana EM, Di Pascuale M, Grueterich M, Solomon A, Tseng SC. Keratolimbal allograft in corneal reconstruction. Eye. 2004;18:406–17
14.. Solomon A, Ellies P, Anderson DF, Touhami A, Grueterich M, Espana EM, et al. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology. 2002;109:1159–66.
- Pellegrini G, Traverson CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–3.









